
Crystallography at IMSERC
Mission
The Crystallography Facility supports Northwestern's research and external users by performing solid-phase, single-crystal, and powder analyses on a variety of samples such as drug candidates, catalysts, organic or inorganic LEDs, and energy storage networks. IMSERC Crystallography staff provide service work and offer training to qualified researchers to solve their own structures and/or collect their own data.
Resources
Please explore the following sections for more details on Crystallography operations at IMSERC:
IMSERC Crystallography staff are available for consultation about any aspects of your crystallographic experiments.Equipment
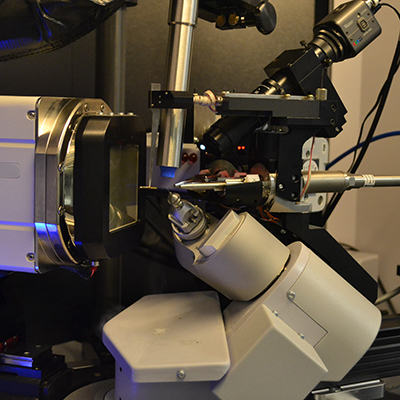
The center currently houses one open Eulerian cradle and four kappa-geometry single-crystal diffractometers, and two powder diffractometers to meet a broad range of crystallographic needs. Most single crystal instruments are equipped with an Oxford Cryosystems nitrogen low-temperature unit, which can run experiments between 80-400K, and up to 500K on one instrument. Additionally, one powder diffractometer has the unique in-situ capabilities to perform measurements from 8 K up to 1,973 K. The IMSERC Crystallography lab is located in BG60/62/66 in Tech.
IMSERC also maintains three high resolution, polarized light microscopes available for student use in the Crystallography facility. Our Nikon SMZ1500 stereozoom and Leica S9i microscopes are equipped with a digital camera and video monitor for visualization of samples. Users can perform visual inspection of their samples with these instruments to assess crystal quality. High resolution photographs can be taken and used for publications or other presentations.
Policies and Safety
Similar safety policies that govern IMSERC govern the Crystallography area policies. You must wear substantial closed-toed shoes and cover unprotected skin whenever possible.
List of Services
- Atomic structure determination from either single crystal or powder diffraction data using X-ray, Neutron, or Synchrotron radiation
- Identification and purity analysis of bulk samples using powder diffraction (coupled with elemental analysis)
- Total scattering methods for the structure determination of nanoparticles and amorphous materials
- Two-dimensional micro-diffraction of samples with small volume (< 1 mm 3)
- In-situ/operando monitoring of reactions, structural/phase transformations, and/or decomposition of materials
- Crystallization and/or co-crystallization of new compounds
Crystallography Cover Highlights


A Hierarchical Nanoporous Diamondoid Superstructure

Best of Chem 2018

A New Horizon for the Mechanical Bond
Efficient Capture of Perrhenate and Pertechnetate by a Mesoporous Zr Metal–Organic Framework and Examination of Anion Binding Motifs

Best Practices for the Synthesis, Activation, and Characterization of Metal–Organic Frameworks

Metal–Organic Framework Supported Cobalt Catalysts for the Oxidative Dehydrogenation of Propane at Low Temperature

Overview of the crystal chemistry of the actinide chalcogenides: incorporation of the alkaline-earth elements

Efficient extraction of sulfate from water using a Zr-metal-organic framework

Chemical, thermal and mechanical stabilities of metal–organic frameworks

Adding to the Arsenal of Zirconium-Based Metal–Organic Frameworks: the Topology as a Platform for Solvent-Assisted Metal Incorporation

High Relaxivity Gd(III)–DNA Gold Nanostars: Investigation of Shape Effects on Proton Relaxation
Designing Higher Surface Area Metal–Organic Frameworks: Are Triple Bonds Better Than Phenyls?

Transmetalation: routes to metal exchange within metal-organic frameworks

ExBox: A Polycyclic Aromatic Hydrocarbon Scavenger

Asararenes—A Family of Large Aromatic Macrocycles

The Role of Polar, Lamdba (Λ)-Shaped Building Units in Noncentrosymmetric Inorganic Structures

Structure-property relationships of porous materials for carbon dioxide separation and capture

Large-scale screening of hypothetical metal–organic frameworks

Lewis Acid Activated Synthesis of Highly Substituted Cyclopentanes by the N-Heterocyclic Carbene Catalyzed Addition of Homoenolate Equivalents to Unsaturated Ketoesters

A coordination chemistry dichotomy for icosahedral carborane-based ligands
Syntheses and characterization of some solid-state actinide (Th, U, Np) compounds

Rational Design, Synthesis, Purification, and Activation of Metal−Organic Framework Materials
