Quantitative NMR
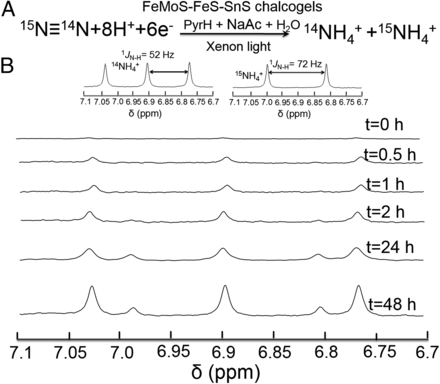
As a intrinsically quantitative technique, NMR can be employed on a large array of applications, among which are reaction monitoring and kinetics. Prof. Kanatzidis group created a novel synthetic chalcogel mimicking nitrogenase that can accomplish photocatalytic N2 fixation and conversion to NH3 at ambient temperature and pressure. They use nmr to quantify the ammonium concentration in their research ( PNAS, 2016 )
Isotope labeling experiment. (A) Simplified equation depicting the photocatalytic isotope N2 reduction experiment. (B) 1H NMR spectra showing the formation of both 14NH4+ and 15NH4+ (corresponding to triplets and doublets, respectively) produced as a function of time from a photocatalytic run using 15N≡14N as isotopic N2 source. (Insets) 1H NMR (400 MHz) spectra of a 14NH4Cl sample and 15NH4Cl sample used as standards in DMSO-d6, respectively.