|
|
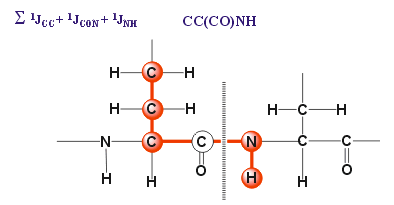
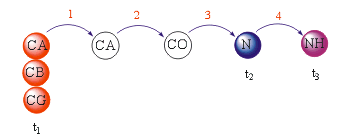
The 3D (H)CC(CO)NH experiment is specifically designed to correlate the 1H and 15N amide resonances of one residue with 13CA and all other 13C side-chain resonances of its preceding residue via the intervening 13CO spin by means of the 1J(NH), 1J(N,CO), 1J(CA,CO) and 1J(C,C) coupling constants. This experiments is closely related to the 3D CBCA(CO)NH experiment.REQUIREMENTS
nh.GIF)
Implementation on AVANCE spectrometers equipped with a third channel. Improved versions using pulsed field gradients (PFGs) are also available and, therefore, in such cases gradient technology is required.VERSIONSThe experiment is applied on 15N,13C-labeled proteins. Because the amide (NH) protons are involved, the (H)CC(CO)NH experiment must be recorded in H2O.
The 3D (H)CC(CO)NH pulse sequence ( 93JMRB114-101 , 93JB225 ) consisted of the following steps:EXPERIMENTAL DETAILS
This experiment is less sensitive than the CBCA(CO)NH experiment because magnetization is distributed intho the whole spin system. Several modified versions have been proposed incorporating the following modifications:
- Initial transfer from 1H to 13C via 1J(CH) using an INEPT pulse sequence.
- 13C chemical shift evolution during a constant-time t1 period of duration 1/(4*J(CC)) followed by a 13C-13C DIPSI-2 pulse train transfers magnetization from all side chain 13C resonances to 13CA.
- Fixed evolution delay to achieve antiphase 13CA magnetization with respect to 13CO via 1J(CA,CO) followed by magnetization transfer to 13CO by simultaneous 90º 13C and 13CO pulses.
- Fixed evolution delay to achieve antiphase 13CO magnetization with respect to 15N via 1J(N,CO) followed by magnetization transfer to 15N by simultaneous 90º 15N and 13CO pulses.
- 15N chemical shift evolution during the constant-time t2 period. Finally, magnetization is transferred back to the NH protons by a retro-INEPT pulse train and proton acquisitionis performed under 15N decoupling.
- A version including WATERGATE:
- A version including 13C-13C cross-polarization transfer but starting from 13C (CC(CO)NH Experiment):
- A version including INEPT transfer but starting from 13C with 1H NOE enhancement: ((H)CCA(CO)NH Experiment):
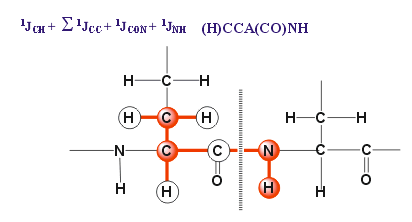
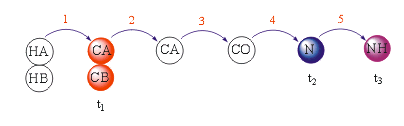
- A version including INEPT transfer but starting from 13C (CCA(CO)NH Experiment):

- A closely related 3D H(CC)(CO)NH experiment in which proton evolution takes place in the t1 period.
- Incorporation of PFG and Constant-time periods ( 95JB211) that can be optimized to identify different amino acid spin systems ( 97JB283 ).
- Use of band-selective cross-polarization schemes ( 96JB147).
- Specific versions have been reported for deuterated proteins ( 96JB59, 96JB351 and 99JB227 ). In such cases, the initial magnetization can be originated from the 13C spins in an analog 3D CC(CO)NH experiment ( 95JACS4187).
- A 3D TROSY-based version has been proposed for a 2H-labeled membrane protein ( 02JB289)
- A related 3D (H)C(C)NH experiment can be also used to obtain sequential conectivities ( 99JB227)
- In overlapped spectra it could be advisable to record a slightly modified 3D (H)CCCO(N)H experiment ( 99PROG93) :
- A 4D HCC(CO)NH experiment has also been proposed ( 92FEBS413 and 93JB349 ).
The 3D (H)CC(CO)NH experiment can be recorded in automation mode.SPECTRA
The (H)CC(CO)NH experiment affords a 3D spectrum in which 1H, 15N and 13C chemical shifts are displayed in three independent dimensions. Sequential connectivities are due to 1J(NH) + 1J(N,CO) + 1J(CA,CO) + n*1J(C,C).RELATED TOPICS
See list of 3D triple-resonance NMR experiments for doubly-labeled proteins.