7.1 HR-MAS of small organic molecules
There is a wide variety of conceivable applications for HR-MAS probes. Among others, one important field for pharmaceutical research concerns combinatorial chemistry.
In combinatorial chemistry, small organic molecules are synthesized on resin beads, consisting of a polymer matrix and a linker material. NMR is advantageous in analyzing these samples since the reactions can be monitored without cleaving off the substrate. High resolution NMR spectra are obtained by swelling the beads in a deuterated solvent in combination with Magic Angle Spinning.
An example from this field is illustrated in figure 7.1. The proton spectrum is obtained from 2 mg of a meta- and para-disubstituted aromatic ring on tentagel resin, swollen in d-chloroform and acquired with a CPMG sequence. The broad resonances of the polymer resin are distinguished from the substrate resonances by exploiting the differences in proton T 2 's using the CPMG sequence as is demonstrated in figure 7.2.

Figure 7.1. 1H hr-MAS NMR spectrum of small organic molecules including a meta- and para-disubstituted aromatic ring on tentagel resin, obtained under the following NMR conditions: DRX400, 2mg in 4mm rotor with spherical spacer, swollen in d-chloroform, cpmg 40msec, 3200Hz spinning, smallest line width at half height 2.7 Hz.
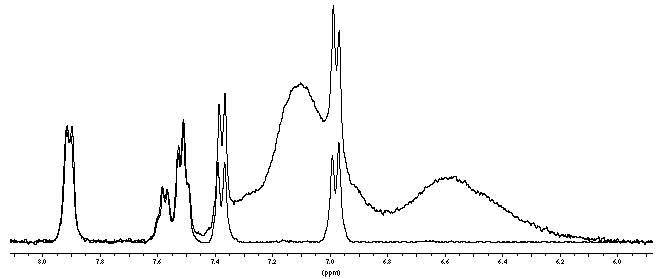
Figure 7.2. Comparison of proton spectra obtained with a single pulse (top) and with a CPMG sequence (bottom). The resonances of the resin are suppressed in the lower spectrum, by employing a CPMG sequence ( --)*n, with =1.5 ms and n=20. Avance 400 MHz, 3200 Hz spin-ning, 2 mg sample on Tentagel substrate, swollen in deuterated chloroform.
Further information on these compounds is obtained from two dimensional NMR techniques. Figures 7.4 and 7.5 show a J-resolved and a double quantum filtered Cosy spectrum of the same compound, respectively.

Figure 7.3. Figure 23: 400 MHz proton spectra of a single 100 m bead (top), separated under the microscope (volume 0.5 nl,1056 scans, weight 600 picograms) and 2mg of the same sample, swollen in CDCl 3 , (8scans). Avance 400, 3200 Hz spinning.
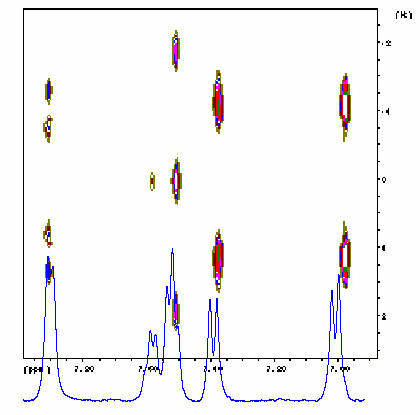
Figure 7.4. 2D J-resolved proton spectrum of 2 mg sample on a Tentagel resin,
swollen in CDCl 3 . Avance 400 MHz, 4 scans, MAS 3200 Hz.

Figure 7.5. Double Quantum filtered Cosy of a small organic molecule
on a Tentagel resin. Avance 400 MHz, 16 scans, 2 mg sample, MAS 3200 Hz.
In
a different application of hr-MAS to the study of small organic molecules,
spots were scratched off a TLC plate and made into a slurry with D 2
O. Figure 7.6 shows the proton spectrum of
approximately 15 g of salicylic acid from a TLC plate.

Figure 7.6. Hr-MAS spectra of TLC-plate spots.
The compound is 15 micrograms of salicylic acid, scratched of the TLC plate and slurried with D 2 O. Avance-600 MHz, 64 scans, 4200 Hz spinning.